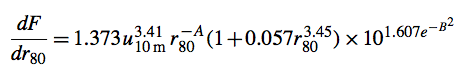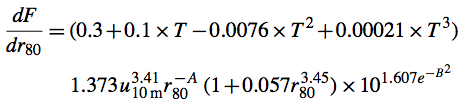Sea salt aerosols
On this page we provide information about sea salt aerosol species used in GEOS-Chem.
Contents
Overview
The treatment of sea salt aerosols in GEOS-Chem has had two major stages of development:
- Original formulation (prior to GEOS-Chem v9-01-03), based on Alexander et al [2005]
- Updated formulation (GEOS-Chem v9-01-03 and higher versions), based on Jaeglé et al, [2011]
--Bob Y. (talk) 16:06, 26 October 2015 (UTC)
Prior Updates to sea salt emissions algorithm
The original reference document for the GEOS-Chem sea salt simulation is Alexander et al [2005]. The updates listed below were added to the original Alexander et al [2005] formulation, in GEOS-Chem versions prior to v9-01-03.
Updated hygroscopic growth factors
Becky Alexander recommends new hygroscopic growth factors for sea salt aerosols. For more information, please see her post on the discussion page.
Modification of size bins for coarse mode aerosols
Lyatt Jaeglé wrote:
- I think that we should change the dry size bins for the coarse mode aerosols in input.geos. Instead of using:
Online SEASALT AEROSOLS : T
=> SALA radius bin [um]: 0.1 0.5
=> SALC radius bin [um]: 0.5 10.0
- we should use a smaller upper cut for the dry radius of the coarse mode aerosols (up to 4 microns dry size ==> 8-10 microns radius for wet sea salt).
Online SEASALT AEROSOLS : T
=> SALA radius bin [um]: 0.1 0.5
=> SALC radius bin [um]: 0.5 4.0
- The first two changes lead to a factor of 2 decrease in total sea-salt emissions (from ~8000 Tg/yr to ~4300 Tg/yr using GEOS-4 winds for 2003 2x2.5). The last change leads to another reduction by 40% in emissions. However the total burden of sea-salt aerosols (~12.5 Tg) remains nearly unchanged compared to the old formulation (~13.7 Tg) because of the strong non-linearity of the dry deposition velocity at sizes > 2 microns. Indeed the lifetime of coarse mode sea-salt aerosols (0.5-4um vs 0.5-10um) increases by a factor of almost 3.
- Here is a summary of the changes:
- Old formulation in GEOS-Chem (2x2.5 GEOS-4 winds 2003)
0.1-0.5 um 0.5-10 um Total: 0.1-10 um Emissions (Tg/yr) 106 7865 7970 Dry deposition (Tg/yr) 4.7 5012 5016 Wet deposition (Tg/yr) 102 2859 2955 Burden (Tg) 0.73 13.01 13.74 Lifetime (hours) 60 14 15
- New formulation, includes the following changes:
- Changing BETHA from 1.0 to 2.0 (as described on the GEOS-Chem v8-02-04 wiki page)
- Changing LOG to LOG10 in the expression for sea salt base emissions (as described on GEOS-Chem v8-02-04 wiki page)
- Capping of the coarse sea salt aerosol bin size at 4um
0.1-0.5 um 0.5-4 um Total: 0.1-4 um Emissions (Tg/yr) 92 2633 2725 Dry deposition (Tg/yr) 4 689 693 Wet deposition (Tg/yr) 87 1944 2031 Burden (Tg) 0.63 11.92 12.55 Lifetime (hours) 60 40 40
- I am also including calculations for a 3rd bin size 4-10 microns
4-10 um Emissions (Tg/yr) 1544 Dry deposition (Tg/yr) 1156 Wet deposition (Tg/yr) 387 Burden (Tg) 1.6 Lifetime (hours) 9
- While these larger aerosols (4-10 microns dry size) add another 50% to the emissions, they only contribute to 12% of the burden because of their short lifetime. So if we want to stick to 2 size bins, I think that it's fine to neglect these larger aerosols and limit the upper cut of the coarse mode sea-salt aerosols to 4 microns.
- The overall sea-salt emissions ~3000 Tg/year is now similar to what other studies found when applying the Monahan formula: Monahan (1986), Spillane et al. (1986), Gong et al. (1998), Penner et al. (2001), etc... This is also within the range recommended by Lewis & Schwartz.
- I also tried the Gong (2003) formulation which leads to a factor of ~2 decrease in emissions of accumulation mode aerosols but little change to the coarse mode aerosols. I am in the process of evaluating the sea-salt formulation against comparisons to cruise sea-salt observations from PMEL and find that both Gong (2003) and Monahan tend to overestimate sea-salt emissions at the high wind speeds in mid-latitudes and underestimate emissions in subtropical warmer waters. I am working on updating the Gong formulation based on SST.
- Note that the optical properties currently used in the GEOS-Chem (jv_spec.dat) assume log-normal size distributions that lead to effective radii that are too large for coarse mode aerosols: ~9 microns at 50% RH! Based on observed sea-salt size distributions, this should be much smaller ~ 1-2 microns. This correction leads to larger AODs due to sea-salt. I think that Colette and Randall are working on updating jv_spec.dat and I will send them my recommendations.
--Bob Y. 10:56, 23 November 2009 (EST)
Recent Updates to sea salt simulation
The GEOS-Chem sea salt simulation was updated following Jaeglé et al. [2011]. These changes were implemented in v9-01-03 (public release 14 Sep 2012) and later versions.
Update molecular weight of sea salt tracers
This update was included in GEOS-Chem v9-02 (public release 03 Mar 2014). This update is included in Adjoint v35j.
Colette Heald wrote:
- We'd like to propose a minor change to input.geos:
- The molecular weight of the sea salt tracers should be listed as 31.4 g/mol (not as 36.0 as current). This is consistent with actual average composition of sea salt, and international guidelines from the IAPWS (http://www.iapws.org/relguide/seawater.pdf). It may be helpful to add a note somewhere to the effect that users should use the molar mass of any individual constituent of interest. While everything in the GEOS-Chem sea salt code is consistently calculated in terms of total mass, this clarification may be helpful for users who might use the MW from input.geos to convert the sea salt concentrations to global burdens, for example.
--Melissa Sulprizio 12:36, 9 December 2013 (EST)
Molecular weight discrepancy in drydep_mod.F
This update was validated with 1-month benchmark simulation v11-01e (approved 04 Jan 2016).
SALA (accumulation mode sea salt) and SALC (coarse mode sea salt) have historically been assigned molecular weights of 36 g/mol. These molecular weights appear to have been used since at least v7-02-03 (Feb 2005). But in GEOS-Chem v9-02 (release date 03 Mar 2015), the molecular weights of SALA and SALC were changed from 36 g/mol to 31.4 g/mol. This was a modification submitted by Lyatt Jaeglé.
However, the implementation of the new molecular weights was inconsistent. For some reason, only the input.geos files were updated with the new molecular weights (= 31.4 g/mol) for SALA and SALC. The XMW variable in GeosCore/drydep_mod.F was still being assigned with the old molecular weight values (= 0.036 kg/mol = 36 g/mol). Therefore, since GEOS-Chem v9-02, dry deposition of sea salt tracers SALA and SALC has been calculated using incorrect molecular weights.
In GEOS-Chem v11-01e, we now obtain the correct molecular weights for SALA and SALC (= 31.4 g/mol = 0.0314 kg/mol) from the GEOS-Chem species database object. These molecular weights are assigned to the XMW variable in GeosCore/drydep_mod.F. This ensures that the same molecular weights for SALA and SALC are now used consistently throughout GEOS-Chem.
--Bob Yantosca (talk) 18:44, 4 January 2016 (UTC)
SST dependent sea salt emissions
This update was tested in the 1-month benchmark simulation v9-01-03d and approved on 12 Jan 2012.
In GEOS-Chem v10-01 and higher versions, sea salt emissions are now handled by the HEMCO emissions component. The algorithm below is now part of the HEMCO module hcox_seasalt_mod.F90.
Sea salt emissions now include both a wind speed and sea surface temperature (SST) dependence. The sea salt source function is based on Gong (2003), which is based on Monahan et al. (1986). The Gong (2003) formulation expresses the density function dF/dr80 (in units of particles m-2 s-1 micrometer-1) as follows:
A and B are parameters depending on r80, the particle radius at RH = 80% (with r80 being close to twice the dry radius of sea salt particles).
Based on a comparison of GEOS-Chem sea salt simulation with coarse mode sea salt mass concentration observations obtained on 6 PMEL cruises, a new SST dependent source function was derived (Jaegle et al., 2011):
where T is the SST expressed in degrees Celsius (valid temperature range: 0-30C).
This new empirical source function leads to improved agreement of GEOS-Chem relative to sea salt mass concentration observations from cruises and ground-based stations, as well as AOD observations from MODIS and AERONET.
Recommended size range for sea salt: Accumulation mode: 0.01-0.5 microns Coarse mode: 0.5 - 8 microns
Note that in Jaeglé et al. (2011) we used 1 accumulation bin (0.01-0.5) and 2 coarse mode bins (0.5-4; 4-10). Due to the non-linearity of dry deposition, using a single coarse bin 0.5-10 microns leads to an overestimate of the sea salt burden, hence we recommend using 0.5-8 microns.
Updates to sea salt dry deposition
This update was tested in the 1-month benchmark simulation v9-01-03d and approved on 12 Jan 2012.
In GEOS-Chem v10-01 and higher versions, Dry deposition is now applied in a single location in the code (in routine DO_TEND of module GeosCore/mixing_mod.F90).
Over land, sea salt dry deposition velocities are calculated using the Zhang et al. (2001) scheme, which is based on the Slinn (1982) model for vegetated canopies. Over the oceans, we have implemented the Slinn and Slinn (1980) deposition model for natural waters. Following the recommendation of Lewis and Schwartz (2004) we assume RH = 98% in the viscous sublayer (0.1-1mm thick layer above the ocean surface). We integrate the dry deposition velocity over each size bin using a bimodal size distribution for sea salt (see below), which includes growth as a function of local RH (see below).
Overall these changes lead to a factor of 3 increase in dry deposition velocity for coarse mode sea salt and a factor of 2 decrease for accumulation mode sea salt.
Updates to hygroscopic growth
The hygroscopic growth of sea salt aerosols is based on Equation (5) in Lewis and Schwartz (2006), which yields more accurate results at RH>98% than the Gerber (1985) formulation previously used in GEOS-Chem.
Updates to optical properties
The size distribution of accumulation mode sea salt aerosols assumes a dry geometric radius rdg=0.085 micrometers with a geometric standard deviation 2.03 micrometers. This is based on cruises in the remote Pacific Ocean (Quinn et al., 1996). For coarse mode sea salt aerosols we use rdg=0.4 micrometers with a geometric standard deviation of 1.8 micrometers based on Reid et al. (2006).
These size distributions are used in the Mie theory calculation of extinction efficiency. They are also used in calculating the size integrated dry deposition velocity of sea salt aerosols.
Overall impact on distribution of sea salt
Implementing these changes leads to small changes in the mean global burdens of accumulation mode (20% decrease) and coarse model (25% increase) sea salt aerosols. However, the spatial changes are much larger, with a 30-50% decrease at high latitudes and a factor of ~2 increase over tropical regions. See media: GC_seasalt_update.pdf for more info.
Comparing modeled sea salt to observations
Please see this discussion about comparing GEOS-Chem modeled sea salt concentrations to observations on the discussion page.
--Bob Y. 12:17, 25 February 2013 (EST)
Computing PM2.5 concentrations from GEOS-Chem output
For information on how to compute particulate matter (PM2.5) from GEOS-Chem diagnostic outputs, please see our Particulate matter in GEOS-Chem wiki page.
--Bob Yantosca (talk) 21:13, 10 February 2016 (UTC)
References
- Alexander, B., R.J. Park, D.J. Jacob, Q.B. Li, R.M. Yantosca, J. Savarino, C.C.W. Lee, and M.H. Thiemens, Sulfate formation in sea-salt aerosols: Constraints from oxygen isotopes, J. Geophys. Res., 110, D10307, 2005. PDF
- Gong, S. L.: A parameterization of sea-salt aerosol source func- tion for sub- and super-micron particles, Global Biogeochem. Cy., 17(4), 1097, doi:10.1029/2003GB002079, 2003.
- Jaeglé, L., P.K. Quinn, T. Bates, B. Alexander, and J.-T. Lin (2011), Global distribution of sea salt aerosols: New constraints from in situ and remote sensing observations, Atmos. Chem. Phys., 11, 3137-3157, doi:10.5194/acp-11-3137-2011.PDF
- Lewis E. R. and Schwartz S. E., Comment on "Size distribution of sea-salt emissions as a function of relative humidity" Atmos. Environ. 40, 588-590 (2006); doi:10.1016/j.atmosenv.2005.08.043
- Quinn, P. K., et al.: Chem- ical and optical properties of marine boundary layer aerosol particles of the mid-Pacific in relation to sources and meteorological transport, optical properties of sea salt aerosols, J. Geophys. Res., 102, 23269–23275, 1996.
- Reid, J. S., et al.: Reconciliation of coarse mode sea-salt aerosol particle size measurements and parameterizations at a sub- tropical ocean receptor site, J. Geophys. Res., 111, D02202, doi:10.1029/2005JD006200, 2006.
- Slinn, W. G. N.: Predictions for particle deposition to vegetative canopies, Atmos. Environ., 16, 1785–1794, 1982.
- Slinn, S. A. and Slinn, W. G. N.: Predictions for particle deposition on natural-waters, Atmos. Environ., 14, 1013–1016, 1980.
- Zhang, L., Gong, S., Padro, J., and Barrie, L.: A size-segregated particle dry deposition scheme for an atmospheric aerosol mod- ule, Atmos. Environ., 35, 549–560, 2001.
Previous issues that have now been resolved
Double-substitution bug in routine GET_ALK
NOTE: This fix was standardized into GEOS-Chem v8-01-02 and higher versions.
Becky Alexander wrote:
- The code in GET_ALK (in routine seasalt_mod.f) as it is now is wrong. I did a substitution twice by mistake, that should have been applied only once. This is calculated for both accumulation and coarse mode seasalt, for both SO2 and HNO3, so there are 4 places in the code that must be fixed.
- The correct code should be as follows:
!----------------------------------
! SO2 uptake onto fine particles
!----------------------------------
! calculate gas-to-particle rate constant for uptake of
! SO2 onto fine sea-salt aerosols [Jacob, 2000] analytical solution
CONST1 = 4.D0/(V*GAMMA_SO2)
A1 = (RAD1/DG)+CONST1
B1 = (RAD2/DG)+CONST1
!-----------------------------------------------------------------------------
! Prior to 7/18/08:
! Becky Alexander's fix to remove double-substitution (bec, bmy, 7/18/08)
! Remove these lines:
! TERM1A = (((B1/DG)**2)+(2.0D0*CONST1*B1/DG)+(CONST1**2)) -
! & (((A1/DG)**2)+(2.0D0*CONST1*A1/DG)+(CONST1**2))
! TERM2A = 2.D0*CONST1*(((B1/DG)+CONST1)-((A1/DG)+CONST1))
! TERM3A = (CONST1**2)*(LOG((B1/DG)+CONST1) -
! & LOG((A1/DG)+CONST1))
! KT1 = 4.D0*PI*N1*(DG**2)*(TERM1A - TERM2A + TERM3A)
!-----------------------------------------------------------------------------
TERM1A = ((B1**2)/2.0d0) - (((A1**2)/2.0d0)
TERM2A = 2.D0*CONST1*(B1-A1)
TERM3A = (CONST1**2)*LOG(B1/A1)
KT1 = 4.D0*PI*N1*(DG**3)*(TERM1A - TERM2A + TERM3A)
!----------------------------------
! SO2 uptake onto coarse particles
!----------------------------------
! calculate gas-to-particle rate constant for uptake of
! SO2 onto coarse sea-salt aerosols [Jacob, 2000] analytical solution
CONST2 = 4.D0/(V*GAMMA_SO2)
A2 = (RAD2/DG)+CONST2
B2 = (RAD3/DG)+CONST2
!------------------------------------------------------------------------------
! Prior to 7/18/08:
! Becky Alexander's fix to remove double-substitution (bec, bmy, 7/18/08)
! Remove these lines:
! TERM1B = (((B2/DG)**2)+(2.0D0*CONST2*B2/DG)+(CONST2**2)) -
! & (((A2/DG)**2)+(2.0D0*CONST2*A2/DG)+(CONST2**2))
! TERM2B = 2.D0*CONST2*(((B2/DG)+CONST2)-((A2/DG)+CONST2))
! TERM3B = (CONST2**2)*(LOG((B2/DG)+CONST2) -
! & LOG((A2/DG)+CONST2))
! KT2 = 4.D0*PI*N2*(DG**2)*(TERM1B - TERM2B + TERM3B)
!------------------------------------------------------------------------------
TERM1B = ((B2**2)/2.0d0) - (((A2**2)/2.0d0)
TERM2B = 2.D0*CONST2*(B2-A2)
TERM3B = (CONST2**2)*LOG(B2/A2)
KT2 = 4.D0*PI*N2*(DG**3)*(TERM1B - TERM2B + TERM3B)
KT = KT1 + KT2
!----------------------------------
! HNO3 uptake onto fine particles
!----------------------------------
! calculate gas-to-particle rate constant for uptake of
! HNO3 onto fine sea-salt aerosols [Jacob, 2000] analytical solution
CONST1N = 4.D0/(V*GAMMA_HNO3)
A1N = (RAD1/DG)+CONST1N
B1N = (RAD2/DG)+CONST1N
!-----------------------------------------------------------------------------
! Prior to 7/18/08:
! Becky Alexander's fix to remove double-substitution (bec, bmy, 7/18/08)
! Remove these lines:
! TERM1AN = (((B1N/DG)**2)+(2.0D0*CONST1N*B1N/DG)+(CONST1N**2)) -
! & (((A1N/DG)**2)+(2.0D0*CONST1N*A1N/DG)+(CONST1N**2))
! TERM2AN = 2.D0*CONST1N*(((B1N/DG)+CONST1N)-((A1N/DG)+CONST1N))
! TERM3AN = (CONST1N**2)*(LOG((B1N/DG)+CONST1N) -
! & LOG((A1N/DG)+CONST1N))
! KT1N = 4.D0*PI*N1*(DG**2)*(TERM1AN - TERM2AN + TERM3AN)
!-----------------------------------------------------------------------------
TERM1AN = ((B1N**2)/2.0d0) - (((A1N**2)/2.0d0)
TERM2AN = 2.D0*CONST1N*(B1N-A1N)
TERM3AN = (CONST1N**2)*LOG(B1N/A1N)
KT1N = 4.D0*PI*N1*(DG**3)*(TERM1AN - TERM2AN + TERM3AN)
!----------------------------------
! HNO3 uptake onto coarse particles
!----------------------------------
! calculate gas-to-particle rate constant for uptake of
! HNO3 onto coarse sea-salt aerosols [Jacob, 2000] analytical solution
CONST2N = 4.D0/(V*GAMMA_HNO3)
A2N = (RAD2/DG)+CONST2N
B2N = (RAD3/DG)+CONST2N
!-----------------------------------------------------------------------------
! Prior to 7/18/08:
! Becky Alexander's fix to remove double-substitution (bec, bmy, 7/18/08)
! Remove these lines:
! TERM1BN = (((B2N/DG)**2)+(2.0D0*CONST2N*B2N/DG)+(CONST2N**2)) -
! & (((A2N/DG)**2)+(2.0D0*CONST2N*A2N/DG)+(CONST2N**2))
! TERM2BN = 2.D0*CONST2N*(((B2N/DG)+CONST2N)-((A2N/DG)+CONST2N))
! TERM3BN = (CONST2N**2)*(LOG((B2N/DG)+CONST2N) -
! & LOG((A2N/DG)+CONST2N))
! KT2N = 4.D0*PI*N2*(DG**2)*(TERM1BN - TERM2BN + TERM3BN)
!-----------------------------------------------------------------------------
TERM1BN = ((B2N**2)/2.0d0) - (((A2N**2)/2.0d0)
TERM2BN = 2.D0*CONST2N*(B2N-A2N)
TERM3BN = (CONST2N**2)*LOG(B2N/A2N)
KT2N = 4.D0*PI*N2*(DG**3)*(TERM1BN - TERM2BN + TERM3BN)
Please make the fix in your version, or you may download it from ftp://ftp.as.harvard.edu/pub/geos-chem/patches/v8-01-01/seasalt_mod.f_w_getalk_fix.
Also see this document by Becky Alexander which describes the analytical solution in more detail.
Duncan Fairlie replied:
- Thanks for taking a look at this. I will look at your code corrections and integrate them into my dust code.
- Since we're looking back at the analytical solution, I think the last line should read
Kt = 4.pi.N.D(cubed)[ ] ,
- the extra factor of D coming from
dr = D.dx,
- and the limits of the integral
( r=[a,b] )
- become
X = [a/D+c, b/D+c]
- I'll recheck my math, and look back at the code.....
--Bob Y. 16:25, 22 February 2010 (EST)