| Table 1A - Bimolecular Singlet O2 |
|---|
| No quantitative updates. |
| Table 1B - Bimolecular HOx |
|---|
| No quantitative updates. |
| Table 1C - Bimolecular NOx |
|---|
| No quantitative updates. |
| Table 1D - Bimolecular Organic |
|---|
| GEOS-Chem Reaction | v10 (JPL 10-6) | JPL 15-10 | Comparison |
|---|
| MACR + O3 = OH + HO2 + HCOOH + CO + MGLY + CH2O | 1.40E-15*exp(-2100/T) | 1.5e-15*exp(-2110/T) |  JPL201510andGCv10_MACRplO3_eq_OHplHO2plHCOOHplCOplMGLYplCH2O.png |
| MVK + O3 = OH + HO2 + HCOOH + CO + ALD2 + MGLY + CH2O | 8.50E-16*exp(-1520/T) | 8.5e-16*exp(-1520/T) |  JPL201510andGCv10_MVKplO3_eq_OHplHO2plHCOOHplCOplALD2plMGLYplCH2O.png |
| MACR + OH = MAO3 + MRO2 | 8.0E-12*exp(380/T) | 9.6e-12*exp(360./T) |  JPL201510andGCv10_MACRplOH_eq_MAO3plMRO2.png |
| MVK + OH = VRO2 | 2.6e-12*exp(610/T) | 2.7e-12*exp(580./T) | 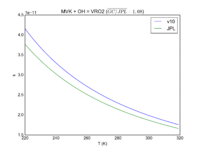 JPL201510andGCv10_MVKplOH_eq_VRO2.png |
| ISOP + OH = RIO2 | 3.1e-11*exp(350/T) | 3.0e-11*exp(360./T) | 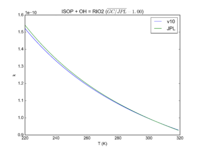 JPL201510andGCv10_ISOPplOH_eq_RIO2.png |
| MACR + NO3 = MAN2 | 2.30e-15 | 3.4e-15 | 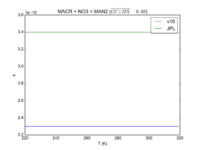 JPL201510andGCv10_MACRplNO3_eq_MAN2.png |
| ISOP + NO3 = INO2 | 3.3E-12*exp(-450/T) | 3.5e-12*exp(-450/T) | 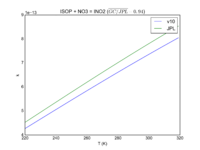 JPL201510andGCv10_ISOPplNO3_eq_INO2.png |
| Table 1F - Bimolecular ClOx |
|---|
| Non CFC/HCFC ClOx needs review. (volunteer by putting your name here) |
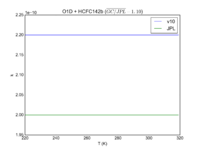
| OH + HCFC22 = Cl + H2O | 1.05E-12*exp(-1600./T) | 9.2e-13*exp(-1560./T) | 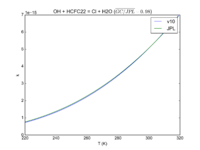 JPL201510andGCv10_OHplHCFC22_eq_ClplH2O.png |
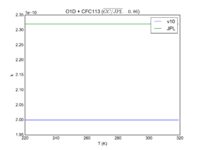
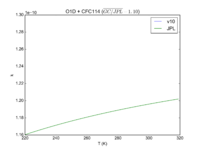
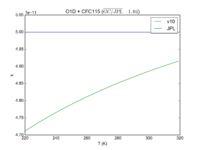
| OH + HCFC123 = Cl + H2O | 6.30E-13*exp(-850./T) | 7.4e-13*exp(-900./T) | 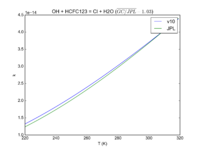 JPL201510andGCv10_OHplHCFC123_eq_ClplH2O.png |
| Table 1G - Bimolecular BrOx |
|---|
| Needs review. (volunteer by putting your name here) |
| Table 1H - Bimolecular IOx |
|---|
| Needs review. (volunteer by putting your name here) |
| Table 1I - Bimolecular SOx |
|---|
| Needs review. (volunteer by putting your name here) |
| Table 2-1 - Termolecular |
|---|
| GEOS-Chem Reaction | v10 (JPL 10-6) | JPL 15-10 | Comparison |
|---|
| HO2 + NO2 + M = HNO4 | GP(A0 = 2.e-31, B0 = 3.4, A1 = 2.9e-12, B1 = 1.1) | GP(A0 = 1.9e-31, B0 = 3.4, A1 = 4e-12, B1 = 0.3) | 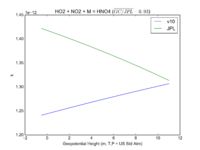 JPL201510andGCv10_HO2plNO2plM_eq_HNO4.png |
| NO2 + NO3 + M = N2O5 | GP(A0 = 2.00E-30, B0 = 4.4E+00, A1 = 1.40E-12, B1 = 7.0E-01) | GP(A0 = 2.4e-30, B0 = 3., A1 = 1.6e-12, B1 = -0.1) | 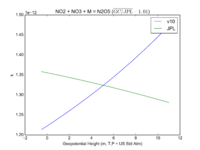 JPL201510andGCv10_NO2plNO3plM_eq_N2O5.png |
| OH + CO + M = H + CO2 | GY(A0 = 5.9e-33, B0 = 1.4e0, A1 = 1.1e-12, B1 = -1.3e0, A2 = 1.5e-13, B2 = -0.6e0, A3 = 2.1e09, B3 = -6.1e0) | GY(A0 = 5.9e-33, B0 = 1., A1 = 1.1e-12, B1 = -1.3e0, A2 = 1.5e-13, B2 = 0., A3 = 2.1e09, B3 = -6.1e0) | 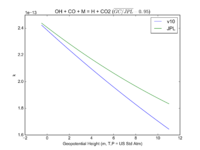 JPL201510andGCv10_OHplCOplM_eq_HplCO2.png |
| OH + PRPE + M = PO2 | GP(A0 = 8.00E-27, B0 = 3.5E+00, A1 = 3.00E-11, B1 = 1.0E+00) | GP(A0 = 4.6e-27, B0 = 4., A1 = 2.6e-11, B1 = 1.3) | 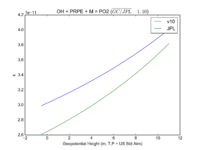 JPL201510andGCv10_OHplPRPEplM_eq_PO2.png |
| ClO + ClO + M = Cl2O2 | GP(1.60E-32, 4.5E+00 , 3.00E-12, 2.0E+00) | GP(A0 = 1.9e-32, B0 = 3.6, A1 = 3.7e-12, B1 = 1.6) | 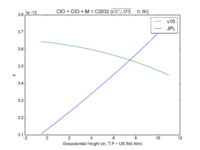 JPL201510andGCv10_ClOplClOplM_eq_Cl2O2.png |
| Table 3-1 |
|---|
| N2O5 = NO2 + NO3 | GP(A0 = 7.40E-04, B0 = 4.4E+00, C0 = -11000., A1 = 5.18E+14, B1 = 7.0E-01, C1 = -11000.) | GP(A0 = 2.4e-30/5.8e-27, B0 = 3., C0 = -10840, A1 = 1.6e-12/5.8e-27, B1 = -0.1, C1 = -10840) |  JPL201510andGCv10_N2O5_eq_NO2plNO3.png |
| HNO4 = HO2 + NO2 | GP(A0 = 2.e-31 / 2.1e-27, B0 = 3.4, C0 = -10900., A1 = 2.9e-12 / 2.1e-27, B1 = 1.1, C1 = -10900.) | GP(A0 = 1.9e-31 / 2.1e-27, B0 = 3.4, C0 = -10900., A1 = 4e-12 / 2.1e-27, B1 = 0.3, C1 = -10900.) |  JPL201510andGCv10_HNO4_eq_HO2plNO2.png |
| 1. Termolecular rates coefficients are evaluated from -0.5km to 11km in the 1976 US Std Atmosphere temperature and pressures |
| 2. GP is short hand for the GEOS-Chem rate form denoted by P in globchem.dat and corresponding to the JPL termolecular rate defined as k_f([M],T) in Section 2.1 |
| 3. GY is short hand for the GEOS-Chem rate form denoted by Y in globchem.dat and corresponding to the JPL termolecular rate defined as k^{ca}_f([M],T) in Section 2.1 |