|
|
| Line 204: |
Line 204: |
| |-valign="top" | | |-valign="top" |
| |colspan="4"|Needs review. (volunteer by putting your name here) | | |colspan="4"|Needs review. (volunteer by putting your name here) |
| | |
|
| |
|
| |} | | |} |
Revision as of 18:12, 17 May 2016
Summary
JPL has released its 18th evaluation of chemical rate coefficients for atmospheric studies (Burkholder et al., 2015)." A new page (Updates in JPL Publication 15-10) is being created to compare rates between GEOS-Chem v10 and JPL Publication 15-10. A similar comparison was done for JPL Publication 10-6 (Updating standard chemistry with JPL 10-6). For each reaction coefficient that was updated, we will note the rate expression currently used in v10, the updated expression in JPL 15-10, and provide a plot of the two rate coefficients. For expressions that are only temperature dependent, rates will be plotted as a function of temperature between 220 K and 320 K. For termolecular reactions, expressions will be plotted as a function of altitude with temperature and pressure following the 1976 US Standard Atmosphere.
J. B. Burkholder, S. P. Sander, J. Abbatt, J. R. Barker, R. E. Huie, C. E. Kolb, M. J. Kurylo, V. L. Orkin, D. M. Wilmouth, and P. H. Wine "Chemical Kinetics and Photochemical Data for Use in Atmospheric Studies, Evaluation No. 18," JPL Publication 15-10, Jet Propulsion Laboratory, Pasadena, 2015 http://jpldataeval.jpl.nasa.gov.
---B. Henderson 2016-05-03 15:25 (EDT)
JPL Updated Rates Compared to GC v10
NOTE: These tables are a work in progress.
Table 1A - Bimolecular Ox
| GEOS-Chem Reaction
|
v10 (JPL 10-6)
|
JPL 15-10
|
Comparison
|
| No quantitative updates |
|
|
|
Table 1A - Bimolecular O1D
| GEOS-Chem Reaction
|
v10 (JPL 10-6)
|
JPL 15-10
|
Comparison
|
| CH3Cl + O1D |
not active |
|
|
| CH3CCl3 + O1D |
not active |
|
|
| O1D + HCFC22 = O + HCFC22 + ClO + Cl
|
1e-10
|
1.02e-10
|
 JPL201510andGCv10_O1DplHCFC22_eq_OplHCFC22plClOplCl.png |
| O1D + HCFC142b
|
2.20E-10
|
2.00E-10
|
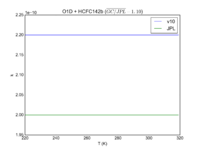 JPL201510andGCv10_O1DplHCFC142b.png |
| O1D + CFC113
|
2.00E-10
|
2.32E-10
|
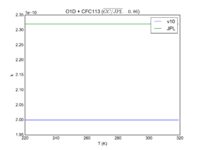 JPL201510andGCv10_O1DplCFC113.png |
| O1D + CFC114
|
1.30E-10
|
1.3E-10*exp(-25/T)
|
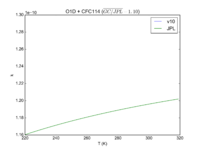 JPL201510andGCv10_O1DplCFC114.png |
| O1D + CFC115
|
5.0E-11
|
5.4E-11*exp(-30/T)
|
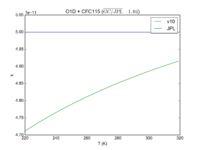 JPL201510andGCv10_O1DplCFC115.png |
Table 1A - Bimolecular Singlet O2
| GEOS-Chem Reaction
|
v10 (JPL 10-6)
|
JPL 15-10
|
Comparison
|
| No quantitative updates |
|
|
|
Table 1B - Bimolecular HOx
| GEOS-Chem Reaction
|
v10 (JPL 10-6)
|
JPL 15-10
|
Comparison
|
| No quantitative updates |
|
|
|
Table 1C - Bimolecular NOx
| GEOS-Chem Reaction
|
v10 (JPL 10-6)
|
JPL 15-10
|
Comparison
|
| No quantitative updates |
|
|
|
Table 1D - Bimolecular Organic
| GEOS-Chem Reaction
|
v10 (JPL 10-6)
|
JPL 15-10
|
Comparison
|
| MACR + O3 = OH + HO2 + HCOOH + CO + MGLY + CH2O
|
1.40E-15*exp(-2100/T)
|
1.5e-15*exp(-2110/T)
|
 JPL201510andGCv10_MACRplO3_eq_OHplHO2plHCOOHplCOplMGLYplCH2O.png |
| MVK + O3 = OH + HO2 + HCOOH + CO + ALD2 + MGLY + CH2O
|
8.50E-16*exp(-1520/T)
|
8.5e-16*exp(-1520/T)
|
 JPL201510andGCv10_MVKplO3_eq_OHplHO2plHCOOHplCOplALD2plMGLYplCH2O.png |
| MACR + OH = MAO3 + MRO2\
|
8.0E-12*exp(380/T)
|
9.6e-12*exp(360./T)
|
 JPL201510andGCv10_MACRplOH_eq_MAO3plMRO2.png |
| MVK + OH = VRO2\
|
2.6e-12*exp(610/T)
|
2.7e-12*exp(580./T)
|
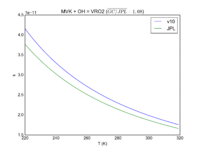 JPL201510andGCv10_MVKplOH_eq_VRO2.png |
| ISOP + OH = RIO2
|
3.1e-11*exp(350/T)
|
3.0e-11*exp(360./T)
|
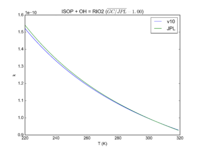 JPL201510andGCv10_ISOPplOH_eq_RIO2.png |
| MACR + NO3 = MAN2
|
2.30e-15
|
3.4e-15
|
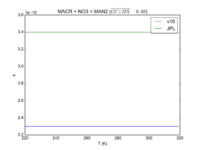 JPL201510andGCv10_MACRplNO3_eq_MAN2.png |
| ISOP + NO3 = INO2
|
3.3E-12*exp(-450/T)
|
3.5e-12*exp(-450/T)
|
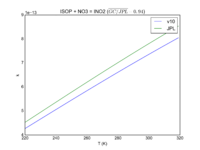 JPL201510andGCv10_ISOPplNO3_eq_INO2.png |
Table 1F - Bimolecular ClOx
| GEOS-Chem Reaction
|
v10 (JPL 10-6)
|
JPL 15-10
|
Comparison
|
| Non CFC/HCFC ClOx needs review. (volunteer by putting your name here)
|
| OH + HCFC22 = Cl + H2O
|
1.05E-12*exp(-1600./T)
|
9.2e-13*exp(-1560./T)
|
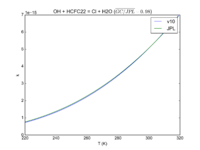 JPL201510andGCv10_OHplHCFC22_eq_ClplH2O.png |
| OH + HCFC123 = Cl + H2O
|
6.30E-13*exp(-850./T)
|
7.4e-13*exp(-900./T)
|
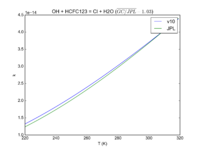 JPL201510andGCv10_OHplHCFC123_eq_ClplH2O.png |
Table 1G - Bimolecular BrOx
| GEOS-Chem Reaction
|
v10 (JPL 10-6)
|
JPL 15-10
|
Comparison
|
| Needs review. (volunteer by putting your name here)
|
Table 1H - Bimolecular IOx
| GEOS-Chem Reaction
|
v10 (JPL 10-6)
|
JPL 15-10
|
Comparison
|
| Needs review. (volunteer by putting your name here)
|
Table 1I - Bimolecular SOx
| GEOS-Chem Reaction
|
v10 (JPL 10-6)
|
JPL 15-10
|
Comparison
|
| Needs review. (volunteer by putting your name here)
|
Table 2-1 - Termolecular
| GEOS-Chem Reaction
|
v10 (JPL 10-6)
|
JPL 15-10
|
Comparison
|
| HO2 + NO2 + M = HNO4
|
GP(A0 = 2.e-31, B0 = 3.4, A1 = 2.9e-12, B1 = 1.1)
|
GP(A0 = 1.9e-31, B0 = 3.4, A1 = 4e-12, B1 = 0.3)
|
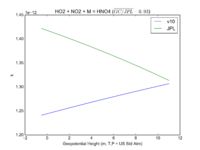 JPL201510andGCv10_HO2plNO2plM_eq_HNO4.png |
| NO2 + NO3 + M = N2O5
|
GP(A0 = 2.00E-30, B0 = 4.4E+00, A1 = 1.40E-12, B1 = 7.0E-01)
|
GP(A0 = 2.4e-30, B0 = 3., A1 = 1.6e-12, B1 = -0.1)
|
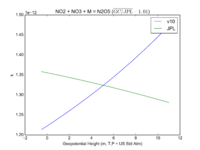 JPL201510andGCv10_NO2plNO3plM_eq_N2O5.png |
| OH + CO + M = H + CO2
|
GY(A0 = 5.9e-33, B0 = 1.4e0, A1 = 1.1e-12, B1 = -1.3e0, A2 = 1.5e-13, B2 = -0.6e0, A3 = 2.1e09, B3 = -6.1e0)
|
GY(A0 = 5.9e-33, B0 = 1., A1 = 1.1e-12, B1 = -1.3e0, A2 = 1.5e-13, B2 = 0., A3 = 2.1e09, B3 = -6.1e0)
|
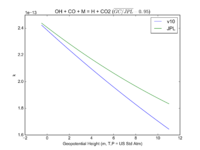 JPL201510andGCv10_OHplCOplM_eq_HplCO2.png |
| OH + PRPE + M = PO2
|
GP(A0 = 8.00E-27, B0 = 3.5E+00, A1 = 3.00E-11, B1 = 1.0E+00)
|
GP(A0 = 4.6e-27, B0 = 4., A1 = 2.6e-11, B1 = 1.3)
|
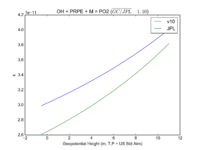 JPL201510andGCv10_OHplPRPEplM_eq_PO2.png |
| ClO + ClO + M = Cl2O2
|
GP(1.60E-32, 4.5E+00 , 3.00E-12, 2.0E+00)
|
GP(A0 = 1.9e-32, B0 = 3.6, A1 = 3.7e-12, B1 = 1.6)
|
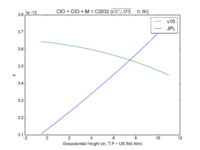 JPL201510andGCv10_ClOplClOplM_eq_Cl2O2.png |
- Termolecular rates coefficients are evaluated from -0.5km to 11km in the 1976 US Std Atmosphere temperature and pressures
- GP is short hand for the GEOS-Chem rate form denoted by P in globchem.dat and corresponding to the JPL termolecular rate defined as k_f([M],T) in Section 2.1
- GY is short hand for the GEOS-Chem rate form denoted by Y in globchem.dat and corresponding to the JPL termolecular rate defined as k^{ca}_f([M],T) in Section 2.1
|
Table 3-1
| GEOS-Chem Reaction
|
v10 (JPL 10-6)
|
JPL 15-10
|
Comparison
|
| N2O5 = NO2 + NO3
|
GP(A0 = 7.40E-04, B0 = 4.4E+00, C0 = -11000., A1 = 5.18E+14, B1 = 7.0E-01, C1 = -11000.)
|
GP(A0 = 2.4e-30/5.8e-27, B0 = 3., C0 = -10840, A1 = 1.6e-12/5.8e-27, B1 = -0.1, C1 = -10840)
|
 JPL201510andGCv10_N2O5_eq_NO2plNO3.png |
| HNO4 = HO2 + NO2
|
GP(A0 = 2.e-31 / 2.1e-27, B0 = 3.4, C0 = -10900., A1 = 2.9e-12 / 2.1e-27, B1 = 1.1, C1 = -10900.)
|
GP(A0 = 1.9e-31 / 2.1e-27, B0 = 3.4, C0 = -10900., A1 = 4e-12 / 2.1e-27, B1 = 0.3, C1 = -10900.)
|
 JPL201510andGCv10_HNO4_eq_HO2plNO2.png |
- GP is short hand for the GEOS-Chem rate form denoted by P in globchem.dat and corresponding to the JPL termolecular rate defined as k_f([M],T) in Section 2.1
- GY is short hand for the GEOS-Chem rate form denoted by Y in globchem.dat and corresponding to the JPL termolecular rate defined as k^{ca}_f([M],T) in Section 2.1
|
Notes
- Skipping CH2OO + ... on 1-93
- Skipping syn-CH3CHOO and anti-CH3CHOO on 1-94
- Skipping FOx reactions
- Skipped ClOx reactions, needs review
- Skipped BrOx reactions, needs review
- Skipped IOx reactions, needs review
- SOx reactions need further consideration perhaps with overlap of Iodine IO and DMS? IO + CH3SCH3 -> products




















